
However, the blueprint’s calculations showed that sequentially administering two tests with that same sensitivity and specificity increases PPV across a wide range of prevalence values at a 5% prevalence, the PPV of two positive tests is 95%. blueprint, at that prevalence a test with 95% sensitivity and 95% specificity would only have a PPV of 50%, rendering its accuracy a crapshoot. While there is still a lot of uncertainty about the prevalence of COVID-19 exposure, a commonly cited estimate for the U.S. But since serological tests are expected to guide decisions such as where to allocate resources and who can more safely return to work, reports of their inaccuracy have raised serious concerns, particularly about the potential for false positives that give individuals a misplaced sense of security (see “Wild West of Antibody Tests”).Ī test’s positive predictive value (PPV) - the probability that a positive result is real - is a function of the prevalence of true positives in a population. The challenges involved in making tests that capture SARS-CoV-2 antibody responses across diverse populations, but don’t cross-react with responses to other viruses, have been made more acute by the COVID-19 crisis’ urgency, which has pushed companies to optimize and validate tests as quickly as possible.įDA has justified its hands-off regulatory approach by arguing that the tests are not used to make clinical diagnoses. But recent policy announcements by CMS and HHS, along with a test validation initiative led by NIH’s National Cancer Institute, indicate the Administration is seeking to improve the quality of COVID-19 antibody tests in the U.S., in part by subjecting them to greater scrutiny (see “New Policies Complicate Regulatory Calculus”). Stenzel is director of FDA’s Office of In Vitro Diagnostics and Radiological Health (see “World Awaits Validation of COVID-19 Serology”).įDA has given companies the option market COVID-19 serological tests without regulatory review. and European markets are failing to meet performance criteria. The double testing strategy, highlighted by FDA’s Timothy Stenzel in a virtual meeting last week, comes as reports indicate the first wave of serological tests flooding U.S. should prioritize testing resources to hot spots, and take advantage of new rapid, point-of-care tests (see “Crisis Spurs Rapid Technologies”).Ī focus of the blueprint was illustrating how administering two sequential COVID-19 serological tests could improve detection of patients previously infected with the virus over single tests administered on their own.
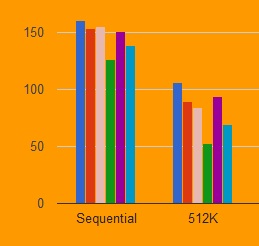
Jointly issued by the White House, CDC and FDA, the blueprint’s core principles include timely and accurate diagnostic testing of every symptomatic patient, sentinel monitoring of at-risk populations, and containment of infections via contact tracing and isolation. The document also divvied up responsibilities across different levels of government and the private sector. But banking on sequential testing to overcome the limitations of individual assays requires keeping paired tests as independent as possible, and an at least two-fold jump in testing capacity. In its COVID-19 Testing Blueprint unveiled Monday, the Trump administration made its latest push to improve the accuracy of serological tests on the U.S.


 0 kommentar(er)
0 kommentar(er)
